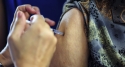
(RxWiki News) A new vaccine approved by the FDA means children may have the opportunity to receive protection from meningitis earlier and without an extra shot.
The US Food and Drug Administration approved the vaccine MenHibrix, which protects against two forms of bacterial meningitis as well as one strain of influenza, on Thursday.
"Be sure to keep your child up to date with immunizations."
MenHibrix, manufactured by GlaxoSmithKline, is short for Meningococcal Groups C and Y and Haemophilus influenza b (Hib) Tetanus Toxoid Conjugate Vaccine. The vaccine was approved for children aged 6 weeks through 18 months.
Although the Hib vaccine, which protects against Haemophilus influenzae type b, is already given to children at their 2 month well-child visit, the current meningitis vaccine is not given until children are preteens or older. This new vaccine combines both for one shot that protect children from meningitis earlier.
Currently, children should receive their first Hib vaccination at 2 months old, followed by boosters at 4 months, 6 months and again at 12 to 15 months, according to the recommended immunization schedule by the Centers for Disease Control and Prevention.
The first meningococcal vaccine is recommended to be given at 11 years old currently, with a booster at 16 years old, but it only protects against group C bacteria.
MenHibrix is designed to be given to children at their 2, 4, 6 and 12 to 15 month well-child visits, just as the current Hib vaccine is.
The new shot would provide additional protection for the meningitis strains without requiring an additional shot.
Although MenHibrix has been approved by the FDA, it will not be added to the CDC recommended schedule of immunizations until the CDC committee reviews the data and decides whether to add it.
Data on the effectiveness and safety of the vaccine came from a clinical trial of 7,521 babies from the US, Mexico, Australia, Belgium and Germany over a seven year period. A total of 3,349 of these children were in the US.
The most common adverse events, or side effects, associated with the vaccine are pain and redness at the injection site, irritability (62 to 71 percent reported), drowsiness (49 to 63 percent), loss of appetite (30 to 34 percent) and fever (11 to 26 percent).
In addition, some premature babies developed apnea, or pauses in breathing, following the injection, so GlaxoSmithKline suggests that parents of preemies talk with their doctor about the risks and benefits of the vaccine before the child receives it.
Any child who has had a serious allergic reaction after receiving any dose of Hib, meningococcal or a tetanus toxoid-containing vaccine, or any ingredient in MenHibrix, should not take it.