(RxWiki News) Stroke treatments have largely focused on how to minimize the level of disability after stroke. And a recent study may have pinpointed a new way to do just that.
Researchers found that a relatively new hands-free ultrasound device, used with a medication often used to break blood clots in stroke patients, was safe and ready for a larger trial.
"Seek treatment for stroke as quickly as possible."
Neurologist Andrew Barreto, MD, of the University of Texas Health Science Center in Houston, was lead author of this study.
Conducted at that Houston facility and the University of Alabama at Birmingham Medical Center, the study enrolled 12 men and eight women with an average of 63 who had had an acute ischemic stroke.
Ischemic strokes account for eight in 10 stroke cases and occur when an artery to the brain is blocked.
The researchers set out to determine the safety of combining intravenously (injected) administered tissue-type plasminogen activator (tPA), a blood-clot buster that the FDA approved in 1996, with two hours of ultrasound.
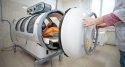
The ultrasound was delivered up to 4.5 hours after the first sign of stroke symptoms by attaching the hands-free ultrasound device to stroke patient's heads. The ultrasound treatment was administered for two hours.
The ultrasonic treatment is used to help break up blood clots and normalize blood flow.
Three months after those treatments, five of the 20 patients were found to have no disability from the stroke, and one patient had a slight disability.
During the same post-treatment period, 13 of the 20 patients went home or into a rehabilitation center for the acutely disabled. Three patients died before they were discharged, and a fourth died later.
Two of the patients had another stroke. One developed an infection that triggered septic shock (complication of infection leading to dangerously low blood pressure).
"Our goal is to open up more arteries in the brain and help stroke patients recover," Dr. Barreto said. "This technology would have a significant impact on patients, families and society if we could improve outcomes by another 5-10 percent by adding ultrasound to patients who've already received tPA."
Earlier ultrasound tools were aimed at patients' heads, according to the researchers. But the new device includes 18 separate probes. It sends ultrasound waves deeper into the brain where bigger blood clots cause the most severe strokes.
One challenge posed by hand-held devices, the researchers wrote, was that there had not been enough ultrasound technicians who were adequately trained to quickly locate the blood clots and, then, hold the tool long and steady enough to optimally break up clots.
"The hands-free device tested in this study represents a leap forward..." the researchers wrote.
"It is a good study [whose] early results are promising," Shanker Dixit, MD, of the Neurology Center of Las Vegas, told dailyRx News.
The study was published online October 24 in Stroke.
The National Institutes of Health funded the study and development of the hands-free device. Several of the study's authors have previously received grants from the National Institute of Neurological Disorders and Stroke, have been paid to consult for medical device makers or patented their own devices.